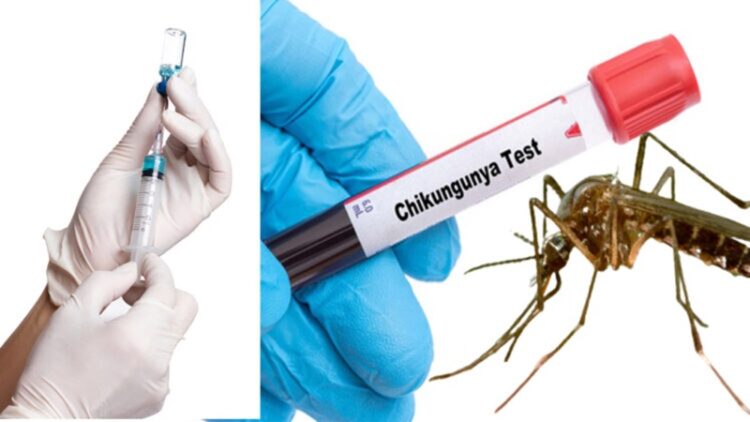
The United States has granted approval for the first-ever vaccine against the Chikungunya virus. On Thursday, US health authorities gave the green light to the vaccine, named Ixchiq and developed by Europe’s Valneva. The Food and Drug Administration (FDA) highlighted the Chikungunya virus as an “emerging global health threat.”
The vaccine, intended for individuals aged 18 and above at an elevated risk of exposure, comes in a single-dose injection. It contains a live, weakened form of the Chikungunya virus, following the standard approach used in other vaccines.
Chikungunya, transmitted by infected mosquitoes, causes symptoms such as fever and severe joint pain. The virus is most prevalent in tropical and subtropical regions of Africa, southeast Asia, and certain parts of the Americas.
Clinical trials involving 3,500 participants were conducted in North America, revealing commonly reported side effects such as headache, fatigue, muscle and joint pain, fever, and nausea.












Comments