The US Food and Drug Administration (FDA) have approved human trials for the brain implant device Neuralink. The agency said that the first clinical trials on humans can commence from now on.
Neuralink is a technological company founded by Elon Musk in 2016, which is developing an implantable brain-computer interface (BCI) that is expected to help in the treatment of neurological disorders and improve cognitive skills in humans.
Earlier, Musk demonstrated “telepathic thinking” by showing a video of a monkey with a Neuralink brain implant using his thoughts to move a cursor of pictures of letters.
The news of human trials of the BCI implant was officially shared by Neuralink via Twitter.



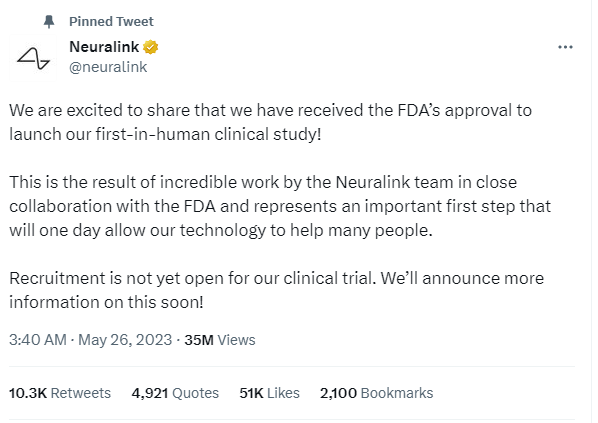











Comments